FAQ
Questions and Answers about Water SoftenersMy water softener (in my home or business) is causing low water pressure – what can I do?
How can a water softener cause low pressure in my house?
Answer #2:
Another occurrence which can take place to cause the low pressure is when the lower collector breaks (the lower collector is attached to the bottom of the distributor tube). This will clog the fixtures and water using appliances. This can be very tumultuous and very expensive. We have heard a story about a cation resin evacuation that caused $5,000.00 in damage.
Here is some more information regarding the cation resin evacuating into the home:
Here is an example of a lower collector which has broken:

September of 2011 our customer ( account #135970) experienced the cation resin flowing into their house when the lower collector broke. This broken lower collector is displayed in the picture above. Troy and Marco responded to this problem. They traveled to this home and cleaned out the cation resin.
Because this problem exists – our company invented a resin screen (this device is shown below)

We have prepared this bypass valve with a metal screen inside. The bypass valve is placed between the water softener (cation resin tank) and the customers home plumbing. This special bypass valve will prevent the cation resin from escaping into the home if the lower collector breaks.
Why rent a water softener rather than buying?
What is the main ingredient of the water softener?
An ion-exchange resin or ion-exchange polymer is an insoluble matrix (or support structure) normally in the form of small (1–2 mm diameter) beads, usually white or yellowish, fabricated from an organic polymer substrate. The material has a highly developed structure of pores on the surface of which are sites with easily trapped and released ions. The trapping of ions takes place only with simultaneous releasing of other ions; thus the process is called ion-exchange. There are multiple different types of ion-exchange resin which are fabricated to selectively prefer one or  several different types of ions. However, most are made of sulfonated cross-linked polystyrene
several different types of ions. However, most are made of sulfonated cross-linked polystyrene
Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification. In many cases, ion-exchange resins were introduced in such processes as a more flexible alternative to the use of natural or artificial zeolites. Also, ion exchange resins are highly effective in the biodiesel filtration process.
Most typical ion-exchange resins are based on crosslinked polystyrene. The required active groups can be introduced after polymerization, or substituted monomers can be used. For example, the crosslinking is often achieved by adding 0.5-25% of divinylbenzene to styrene during the polymerization process. Non-crosslinked polymers are used only rarely because they are less stable. Crosslinking decreases the ion-exchange capacity of the resin and prolongs the time needed to accomplish the ion exchange processes. Particle size also influences the resin parameters; smaller particles have a larger outer surface but cause a larger head loss in the column processes.
Reference: https://en.wikipedia.org/wiki/Ion-exchange_resin
This is a microscopic image of cation resin (the main cleaning ingredient of the water softener)
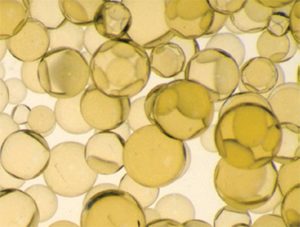
What type and quality of cation resin is the best for the Phoenix Arizona area?
Polystyrene cross-linked with a minimum of 8% Divinylbenzene
C108DQ – Na, WQA/NSF-44 Certified
SOLVENT-FREE (BY STEAM-RINSE) STRONG ACID CATION EXCHANGE RESIN
(Designed for use in water softening applications)
This is the link to the datasheet ‘if you would like specific information on this cation resin’:
How does a Water Softener work?
Will a Water Softener make my water safe to drink?
Why does soft water feel slimy or slick in the shower?
When do the resins in the softener tank need to be changed?
I see ads for “No Salt” needed water conditioners. How do they work without using salt?
1. Many dealers will advertise a no salt water conditioner in a misleading way. Any brand of water conditioner can be operated without using salt. This is done by using a salt substitute, potassium chloride. It is generally more expensive compared to regular salt (sodium chloride), and can be difficult to find in some areas. Also, it is generally recommended you increase the salt setting on your control valve by about 10%, when using a salt substitute. This is usually not the method being referred to as a ”No Salt” water softener today, but be sure!
2. NEW TECHNOLOGY SALT FREE WATER SOFTENERS are a recent and reliable alternative that make perfect sense in most applications. There are multiple methods (many products & claims are hype & a waste of money) however, the only reliable one is a process called template assisted crystallization (TAC).
TAC is a process in which calcium ions in the water are converted to calcium crystals. These crystals now lose any binding, or scaling ability and are washed down the drain w/ the rest of the water. Any residential, industrial, or commercial setting will benefit substantially with one of these systems.
They stop scale build up in the water system and appliances. These systems also eliminate any salt costs and save a considerable amount of space. Additionally, they do not require a control valve and because of this there is no wasted backwash water, and there is very little maintenance.
We specialize in this new technology.
How often do I need to add salt to the Brine Tank?
How much salt should my softener use?
2. We sell only metered valves since they tend to use less salt than a non-metered unit (i.e. one set to regenerate every so many days with no regards for actual water used).
3. The national average is 60 lbs. per month, but that can vary depending upon the quantity and the quality of water being treated.
What kind of salt do you recommend using and do your softeners also use Potassium Chloride in place of salt?
My valve appears to be operating but the salt is not going down. What could cause this problem?
1. Valve is not regenerating due to a mechanical problem.
2. Salt may be bridged (become solid) above water that is at the bottom of the salt tank.
3. If you have been using pellet salt for many years you could have a lot of undissolved residue at the bottom. This residue will not dissolve and also can block water flow in and out of the salt tank.
4. The valve could be failing to draw the brine solution out and if you have a float shut off in the brine tank, it would be prevent the salt tank from overflowing (which it would do if the float shut off was not there).
5. The brine refill control could be clogged, prevent water to refill the salt tank.
(Note: It is highly recommended that you contact an experienced water quality specialist to trouble shoot any problem with your equipment.)
10. I have a working Water Softener, but I am still getting
I have a working Water Softener, but I am still getting Iron Staining. Why is that?
1. It is critical that your system never run empty of salt.
2. It is important that the time of day be kept correct and that no one uses water between 2 a.m. – 3 a.m. when the system is regenerating. While the system is in regeneration, any water used would be unconditioned (coming straight from the well).
3. It could be your resin tank is too small to handle all the iron.
A. What size is the resin tank?
B. What is the level of Iron and Hardness of the water?
4. It could be you are not regenerating often enough, or using enough salt per regeneration.
A. How often does your softener regenerate?
B. How many people are using the water? C. How much salt are you using per month?
5. It could be that your iron content exceeds the recommended maximum. (1 cu.ft. of resin can effectively remove up to 3 parts per million iron without additional treatment.)
6. On rare occasions the iron could be coming from just the hot water tank. If it is more than 20 years old it could be rusting out on the inside, thus putting iron back into the water. This is also true in older homes, again over 20 years old, that used galvanized plumbing.
Above are the common reasons a working water softener might still be allowing you to get staining. For additional help and recommendations, call or contact an experienced water quality specialist.
I have a Water Softener, but I still have odor in my water. Why is that?
1. Odors are typically caused by hydrogen sulfide (“rotten egg smell”) in wells or “bleach” smell in chlorine-treated water; both of these causes can be resolved using an activated carbon filter in conjunction with a water softener.
2. The self-sacrificing rod installed in your hot water heater can sometimes be the cause of your odor in the hot water. Having a qualified plumber replace this rod could solve this problem.
I have very hard water and high Iron. What kind of softener do I need?
How Can I find out what is in my water…or where can I have My Water Tested?
How can I determine what kind of unit, and what size I will need?
How can I tell what my flow rate is?
What kinds of Iron could be in my water?
* Oxidized Iron contains red particles easily visible as the water is drawn from the faucet.
* Soluble or “Clear Water Iron” is very common, and will develop red particles in the water after water is drawn from the faucet, and is exposed to the air for a period of time. The iron particles actually “rust” once they are exposed to air.
* Colloidal Iron consists of extremely small particles of oxidized iron particles suspended in water. This type iron looks more like cloudy, colored water, instead of being able to actually see small red particles of iron. This type iron will not filter well because of the extremely small particle size. (Chlorination may be required).
* Bacterial Iron consists of living organisms found in the water and piping of the well and house. You can tell if you have Bacterial Iron by looking in your toilet flush tank, and finding a reddish/green slime buildup. To confirm this, you should take a sample of this slime to your local health department for testing. This kind of iron is the hardest to get rid of. To completely eliminate this form of bacterial iron requires chlorination of the entire water system, starting with the well casing, well pump, pressure tank and the home plumbing system. (Chlorination may be required).
* Hydrogen Sulfide causes water to have a pungent “rotten egg” odor, and is easily removed using a Manganese Greensand filer.
Can the softener cause pressure loss, if so what do I look for, and what do I need to fix it?
1. On well water, this is usually due to fine sand coming from the well.
2. On softeners installed in the open sunlight (mostly in Florida), a layer of algae can grow and thick pieces of this growth clog the lower distributor tube screen when they start peeling off the inside of the resin tank.
3. On chlorinated water supplies, sand can get into the tank from new construction or work on water lines in the area. All of these situations are rare.
4. The most common cause of pressure loss occurs on chlorinated water. The resin can be damaged by high chlorine levels and turn to mush. This has the same effect as having fine sand at the bottom of the resin tank.
The solution for all of the above problems is to dump the resin tank, clean and rebed with new resins. One cubic foot of softening resins is enough to properly fill the average residential softener. We can calculate the amount for you, if you provide exact resin tank dimensions.
What is meant by scaling or fouling?
Other terms used in the literature to describe fouling include : deposit formation, encrustation, scaling, scale formation, crudding, and deposition. The last four terms are less inclusive than fouling; therefore, they should be used with caution.
Fouling phenomena are common and diverse, ranging from fouling of ships, natural surfaces in the marine environment (marine fouling), fouling of heat-transferring components through ingredients contained in the cooling water or gases, and even the development of plaque or calculus on teeth, or deposits on solar panels on Mars, among other examples.
This article is mostly devoted to the fouling of industrial heat exchanger systems, although the same theory is generally applicable to other varieties of fouling. In the cooling technology and other technical fields, a distinction is made between macro fouling and micro fouling. Of the two, micro fouling is the one which is usually more difficult to prevent and therefore more important.
Components subject to fouling
The following lists examples of components that may be subject of fouling and the direct effects of fouling:
•heat exchanger surfaces – reduces thermal efficiency, increases temperature, creates corrosion, increases use of cooling water
•piping, flow channels – reduces flow, increases pressure drop, increases energy expenditure, may create flow oscillations
•ship hulls – increases fuel usage, reduces the maximum speed
•turbines – reduces efficiency, increases the probability of failure
•solar panels – decreases the electrical power generated
•reverse osmosis membranes – reduces the efficiency of water purification, increases pressure drop, increases energy expenditure
•electrical heating elements – increases the temperature of the element, increases corrosion, reduces the lifespan
•nuclear fuel in pressurized water reactors – axial offset anomaly
•injection/spray nozzles (e.g., a nozzle spraying a fuel into a furnace) – incorrect amount injected, malformed jet, component inefficiency, component failure
•venturi tubes, orifice plates – inaccurate or incorrect measurement of flow rate
•pitot tubes in airplanes – inaccurate or incorrect indication of airplane speed
•teeth – promotes tooth disease, decreases aesthetics
Macro fouling
Macro fouling is caused by coarse matter of either biological or inorganic origin, for example, industrially produced refuse. Such matter enters into the cooling water circuit through the cooling water pumps from sources like the open sea, rivers or lakes. In closed circuits, like cooling towers, the ingress of macro fouling into the cooling tower basin is possible through open canals or by the wind. Sometimes, parts of the cooling tower internals detach themselves and are carried into the cooling water circuit. Such substances can foul the surfaces of heat exchangers and may cause deterioration of the relevant heat transfer coefficient. They may also create flow blockages, redistribute the flow inside the components, or cause fretting damage.
Examples
•Manmade refuse
•Detached internal parts of components
•Algae
•Mussels
•Leaves, parts of plants up to entire trunks
Micro fouling
As to micro fouling, distinctions are made between:
•Scaling or precipitation fouling, as crystallization of solid salts, oxides and hydroxides from water solutions, for example, calcium carbonate or calcium sulfate.
•Particulate fouling, i.e., accumulation of particles, typically colloidal particles, on a surface
•Corrosion fouling, i.e., in-situ growth of corrosion deposits, for example, magnetite on carbon steel surfaces
•Chemical reaction fouling, for example, decomposition or polymerization of organic matter on heating surfaces
•Solidification fouling – when components of the flowing fluid with high-melting-point freeze onto a subcooled surface
•Bio fouling, like settlements of bacteria and algae
•Composite fouling, whereby fouling involves more than one foulant or fouling mechanism.
Precipitation fouling
Temperature dependence of the solubility of calcium sulfate (3 phases) in pure water.
Scaling or precipitation fouling involves crystallization of solid salts, oxides, and hydroxides from solutions. These are most often water solutions, but non-aqueous precipitation fouling is also known.
Through changes in temperature, or solvent evaporation or degasification, the concentration of salts may exceed the saturation, leading to a precipitation of salt crystals. Precipitation fouling is a very common problem in boilers and heat exchangers operating with hard water and often results in limescale.
The calcium carbonate that has formed through this reaction precipitates. Due to the temperature dependence of the reaction, and increasing volatility of CO2 with increasing temperature, the scaling is higher at the hotter outlet of the heat exchanger than at the cooler inlet. In general, the dependence of the salt solubility on temperature or presence of evaporation will often be the driving force for precipitation fouling. The important distinction is between salts with “normal” or “retrograde” dependence of solubility on temperature. The salts with the “normal” solubility increase their solubility with increasing temperature and thus will foul the cooling surfaces. The salts with “inverse” or “retrograde” solubility will foul the heating surfaces. An example dependence of the solubility on temperature is shown in the figure. Calcium sulfate is a common precipitation foulant of heating surfaces due to its retrograde solubility.
Precipitation fouling can also occur in absence of heating or vaporization. For example, calcium sulfate decreases it solubility with decreasing pressure. This can lead to precipitation fouling of reservoirs and wells in oil fields, decreasing their productivity with time.[1] Similarly, precipitation fouling can occur on mixing of incompatible fluid streams.
The following lists some of the industrially most common phases of precipitation fouling deposits observed in practice to form from aqueous solutions:
•Calcium carbonate (calcite, aragonite usually at t > ~50 °C, or rarely vaterite);
•Calcium sulfate (anhydrite, hemihydrate, gypsum);
•Calcium oxalate (e.g., beer stone)
•Barium sulfate;
•Magnesium hydroxide (brucite);
•Silicates (serpentine, acmite, gyrolite, gehlenite, amorphous silica, quartz, cristobalite, pectolite, xonotlite);
•Aluminium oxide hydroxides (boehmite, gibbsite, diaspore, corundum);
•Aluminosilicates (analcite, cancrinite, noselite);
•Copper (metallic copper, cuprite);
•Phosphates (hydroxyapatite);
•Magnetite from extremely low-iron water.
Particulate fouling
Fouling by particles suspended in water (“crud”) or in gas progresses by a mechanism different than precipitation fouling. This process is usually most important for colloidal particles, i.e., particles smaller than about 1 μm in at least one dimension (but which are much larger than atomic dimensions). Particles are transported to the surface by a number of mechanisms and there they can attach themselves, e.g., by flocculation or coagulation. Note that the attachment of colloidal particles typically involves electrical forces and thus the particle behavior defies the experience from the macroscopic world. The probability of attachment is sometimes referred to as “sticking probability”, which for colloidal particles is a function of both the surface chemistry and the local thermo hydraulic conditions. Being essentially a surface chemistry phenomenon, this fouling mechanism can be very sensitive to factors that affect colloidal stability, e.g., zeta potential. A maximum fouling rate is usually observed when the fouling particles and the substrate exhibit opposite electrical charge, or near the point of zero charge of either of them. With time, the resulting surface deposit may harden through processes collectively known as “deposit consolidation” or, colloquially, “aging”.
The common particulate fouling deposits formed from aqueous suspensions include :
•iron oxides and iron oxyhydroxides (magnetite, hematite, lepidocrocite, maghemite, goethite);
•Sedimentation fouling by silt and other relatively coarse suspended matter.
Corrosion fouling
Corrosion deposits are created in-situ by the corrosion of the substrate. They are distinguished from fouling deposits, which form from material originating ex-situ. Corrosion deposits should not be confused with fouling deposits formed by ex-situ generated corrosion products. Corrosion deposits will normally have composition related to the composition of the substrate. Also, the geometry of the metal-oxide and oxide-fluid interfaces may allow practical distinction between the corrosion and fouling deposits. An example of corrosion fouling can be formation of an iron oxide or oxyhydroxide deposit from corrosion of the carbon steel underneath.
Chemical reaction fouling
Chemical reactions may occur on contact of the chemical species in the process fluid with heat transfer surfaces. In such cases, the metallic surface sometimes acts as a catalyst. For example, corrosion and polymerization occurs in cooling water for the chemical industry which has a minor content of hydrocarbons. Systems in petroleum processing are prone to polymerization of olefins or deposition of heavy fractions (asphaltenes, waxes, etc). High tube wall temperatures may lead to carbonizing of organic matter. Food industry, for example milk processing, also experiences fouling problems by chemical reactions.
Fouling through an ionic reaction with an evolution of an inorganic solid is commonly classified as precipitation fouling (not chemical reaction fouling).
Solidification fouling
Solidification fouling occurs when a component of the flowing fluid “freezes” onto a surface forming a solid fouling deposit. Examples may include solidification of wax (with a high melting point) from a hydrocarbon solution, or of molten ash (carried in a furnace exhaust gas) onto a heat exchanger surface. The surface needs to have a temperature below a certain threshold; therefore, it is said to be subcooled in respect to the solidification point of the foulant.
Bio fouling or biological fouling is the undesirable accumulation of micro-organisms, algae and diatoms, plants, and animals on surfaces, for example ships’ hulls, or piping and reservoirs with untreated water. This can be accompanied by microbiologically influenced corrosion (MIC).
Bacteria can form biofilms or slimes. Thus the organisms can aggregate on surfaces using colloidal hydrogels of water and extracellular polymeric substances (EPS) (polysaccharides, lipids, nucleic acids, etc). The biofilm structure is usually complex.
Bacterial fouling can occur under either aerobic (with oxygen dissolved in water) or anaerobic (no oxygen) conditions. In practice, aerobic bacteria prefer open systems, when both oxygen and nutrients are constantly delivered, often in warm and sunlit environments. Anaerobic fouling more often occurs in closed systems when sufficient nutrients are present. Examples may include sulfate-reducing bacteria (or sulfur-reducing bacteria), which produce sulfide and often cause corrosion of ferrous metals (and other alloys). Sulfide-oxidizing bacteria (e.g., Acidithiobacillus), on the other hand, can produce sulfuric acid, and can be involved in corrosion of concrete.
Composite fouling
Composite fouling is common. This type of fouling involves more than one foulant or more than one fouling mechanism working simultaneously. The multiple foulants or mechanisms may interact with each other resulting in a synergistic fouling which is not a simple arithmetic sum of the individual components.
